Establishing lineage tracing models to study the cellular plasticity of metastatic tumor cells
EMT Lineage Tracing Models
Epithelial to mesenchymal transition (EMT), a transdifferentiation program that was originally characterized in embryo development, is also hijacked by tumor cells. Through EMT, epithelial tumor cells lose their polarity and connection with neighboring cells, gain mobility and invasiveness, exhibit resistance to apoptosis, and retrieve stemness properties. These EMT-associated features could adequately meet the requirements for metastasis. EMT has thus been enthusiastically proposed as an essential mechanism for tumor metastasis. However, evidence remains inconclusive as metastases closely resemble the epithelial phenotype of primary tumors. This observation is tentatively explained by the dynamic nature of the EMT process. No sooner than the mesenchymal tumor cells seed at metastatic sites, they regain epithelial phenotypes by undergoing a reverse process of EMT, namely mesenchymal to epithelial transition (MET). Therefore, it would be EMT plasticity, the ability to change their phenotype, but not either epithelial or mesenchymal phenotype itself, that facilitates tumor cells to metastasize.
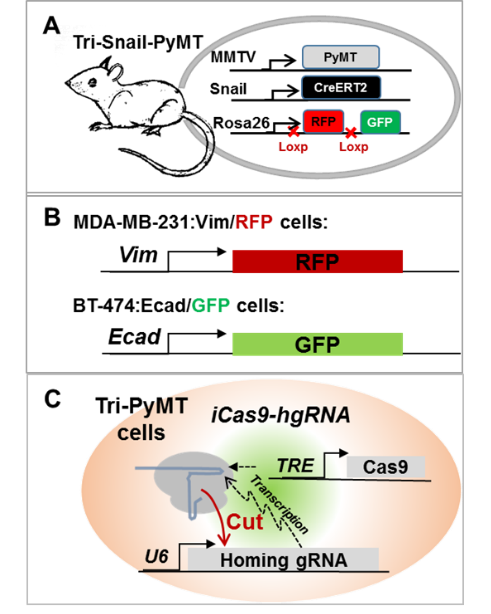
To obtain in vivo evidence of the EMT plasticity in metastasis, we established an EMT lineage tracing model in a multiple-transgenic mouse (MMTV-PyMT:Fsp1-Cre:Rosa26mT/mG, Tri-PyMT). In this model, breast tumor cells that underwent EMT would irreversibly switch their expression of the fluorescence from RFP+ to GFP+ due to the mesenchymal-specific Cre expression. Surprisingly, we found that metastatic lesions did not convert to GFP+. Lung metastases were predominantly composed of RFP+ tumor cells persistently exhibiting epithelial phenotypes. Although the post-EMT tumor cells (GFP+) were detected in the primary tumor, blood, and metastatic lungs, they were significantly outnumbered by their epithelial counterparts. Importantly, we also found that the post-EMT tumor cells exhibited resistance to chemotherapy, significantly contributed to recurrent lung metastases after chemotherapy. These findings pointed to the complexity of EMT contributions in tumor progression. Resolving the controversies in the field will not only improve our mechanistic understanding of metastasis but also provide novel targets/ opportunities in combatting the deadly disease.
Given the transient, reversible and dynamic natures of EMT, we are developing multiple lineage tracing models: 1) Snail-CreERT2 mediated fluorescence switch model to trace EMT events during metastasis; 2) Knock-in fluorescence tag with endogenous EMT marker genes to trace the dynamic changes of EMT status; 3) Revolution barcoding model with CRISPR/home guiding RNA to trace the evolutionary relationships of metastatic tumor cell colonies.

